Surgical Mesh – know the facts.
For centuries surgeons have repaired damaged tissue with sutures of one kind or another. Sutures are, along with scalpels, probably the most fundamental tools of the trade. Suture materials have evolved dramatically, ranging from natural fibres to sophisticated synthetic polymers designed for specific surgical needs. Their selection is guided by a combination of tensile strength, absorption profile, biocompatibility, handling characteristics, and gauge. No matter how good the materials might be, they still have their limitations and their breaking points. In certain circumstances, like reinforcing a weakened area or supporting an organ that has lost its supporting structures, sutures are just not sufficient to get the job done. This is when we turn to a surgical mesh. We do so because a broken suture is a failure of the repair. The easiest analogies to understand are those perhaps of a tennis racket or a cheese wire: if a string breaks on a tennis racket, it becomes useless to the player, and if a wire is pressed too firmly into cheese, it slices down into the flesh of the cheese. For the human body part that needs reinforcement or support, sutures might not provide enough help. This prompted the development of surgical mesh.
The earliest use of mesh-like materials in surgery dates back to the late 19th and early 20th centuries, when surgeons experimented with metal wires, silk and “catgut”. Catgut was not made from cats’ guts! It was made from beef tendons or from the intestinal collagen of sheep, goats or cattle. These early attempts, though innovative, were plagued by infection and rejection of the foreign material due to poor biocompatibility. The landmark turning point came in the 1950s with the development of synthetic polymers such as polypropylene, which offered durability, flexibility and relative inertness when implanted into the human body.
Polypropylene mesh gained widespread acceptance in the 1960s for hernia repairs, where it demonstrated reduced recurrence rates compared to traditional suture-only techniques. This method of hernia repair, popularised by Dr. Irving Lichtenstein in the 1980s, firmly established mesh as the gold standard of hernia care, minimising patients’ post-operative pain and improving their recovery from surgery. Over the following decades, mesh products diversified in form and function. Beyond hernia repair, meshes were adapted for pelvic organ prolapse, incontinence of urine and abdominal reconstruction. New designs incorporated absorbable polymers like polyglycolic acid and polylactic acid, offering temporary support as tissue healed. Lightweight and composite meshes were developed to balance strength with reduced inflammatory response.
What is mesh and how does it help you?
The easiest concept to grasp is that of reinforcement: meshes are designed to reinforce parts of the anatomy that lack integrity or strength. Think of a hole in a jacket sleeve – in the old days we could darn it with a needle and thread. We could do so similarly if we had a hole in human tissue and used suture thread. But when the thread broke, the hole would reappear. An alternative to a darn for the hole in the jacket could be a leather patch sewn over the hole. This might work well for a period of time, but leather perishes and it isn’t a living tissue, so it too might fail over time and the hole would reappear. In the human body the solution would be to fabricate a patch, but make it in such a way that the snapping of one part of the suture wouldn’t matter. Think then of a fishing net with each thread knotting into another. This would ensure longevity, but to give it really good longevity, we would want it to become a living structure that could remodel itself and replenish itself over time.
Mesh is therefore a woven patch, made from suture materials, designed a bit like a fishing net, but one that allows the body’s own healing tissue (“scar tissue” made of fibroblast cells) to grow into the gaps in the net to ultimately reinforce the area with a sheet of living human tissue. This process is important for stimulating collagen deposition, which is a key step in the formation of strong and durable tissue, contributing to long-term structural integrity. You could call mesh a mold for growing your own natural “patch” repair.
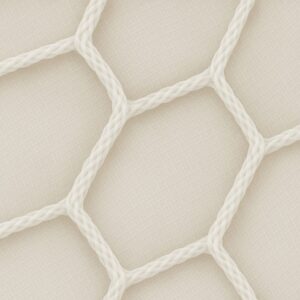
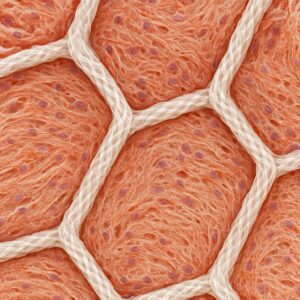
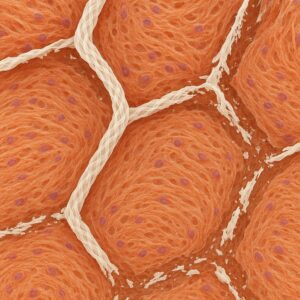
Mesh before implantation Growth of living tissue into the mesh gaps Dissolving mesh
Meshes in use today come in a variety of different materials and surgeons choose their preferred materials depending on the circumstances of the case. At times we might use a “biological” mesh and at other times a “synthetic” mesh. Even within these categories there are subcategories that vary in origin, thicknesses of fibres, stretchability, rates of dissolving, sizes of pores (gaps) in the net, tensile strength, monofilament threads versus braided threads etc. There are many choices at our disposal and a huge body of medical data to inform our choice for a particular patient.
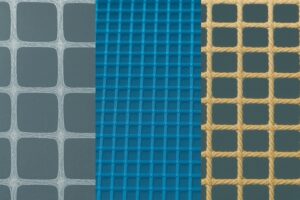
Monofilament (left) and Braided (right) of varying pore sizes
For many patients, what really matters to them is that the surgeon chooses a SAFE and an EFFECTIVE mesh to get the job done. Nobody wishes to have a re-do operation because the repair failed, and nobody wants to have complications if these can be avoided. In my experience, patients like to know a little bit about mesh (particularly as there has been bad press about these in gynaecology and urology) but they largely trust the surgeon to make a good choice on their behalf.
If you’d like to know more about the technical details of meshes and suture materials, read the addendum “More about meshes” below.
If you’d like to know more about the controversies around mesh used in gynaecology and urology, or conversely want to see the independent advice endorsing them for hernia and rectal prolapse surgery, read the addendum “Mesh – the good and the bad” below.
I encourage you to talk to your surgeon about these topics if you are being considered for mesh surgery but have any reservations about the use of mesh in your operation. Peace of mind is essential to a happy outcome for both parties!
MORE ABOUT MESHES
Classification and Material Types
Sutures are broadly classified as absorbable or non-absorbable, and as monofilament or multifilament (braided). Absorbable sutures break down naturally in the body through enzymatic or hydrolytic processes, while non-absorbable sutures are intended to remain in place indefinitely or until removed.
Absorbable Sutures
Natural absorbable sutures include:
- Catgut: Derived from purified collagen of bovine or ovine origin. It is enzymatically degraded, often within 7–21 days, and is rarely used today due to variability of degradation and tissue reaction.
Synthetic absorbable sutures are more predictable and widely used:
- Polyglycolic acid (PGA) and Polyglactin 910 (Vicryl): Braided polymers with good tensile strength and predictable absorption (fully absorbed by 60–90 days).
- Polydioxanone (PDS): A monofilament suture with prolonged strength, often used in deeper tissues; absorption completes around 180 days.
- Poliglecaprone 25 (Monocryl): A monofilament with excellent pliability, used for subcuticular closures and soft tissues, with rapid absorption (complete by 90–120 days).
Non-Absorbable Sutures
These sutures provide long-term or permanent support:
- Silk: A braided natural fibre with excellent handling but moderate tissue reactivity. Though technically non-absorbable, it gradually degrades over years.
- Nylon (Ethilon) and Polypropylene (Prolene): Synthetic monofilaments with high tensile strength and minimal tissue reactivity, ideal for skin closure and vascular surgery.
- Polyester (Ethibond): Braided and coated to reduce tissue drag; often used in cardiovascular and orthopedic procedures.
- Stainless Steel: Extremely strong and biologically inert, used in sternal closures and bone repair.
Gauge and Strength
Suture sizes are measured by the United States Pharmacopeia (USP) system, where higher numbers of zeros indicate finer sutures. For example, 7-0 is finer than 3-0. Choice depends on the tissue type: delicate areas like ophthalmic or microvascular work use 8-0 or 10-0 sutures, while abdominal fascia may require 0 or 1-0 for adequate strength.
Tensile strength retention is critical: absorbable sutures are chosen based on how long they must support healing tissue. For instance, PDS retains 60% of its strength at 4 weeks, suitable for slow-healing tissues, while Monocryl retains strength for only 1–2 weeks
Synthetic Mesh Types
- Polypropylene (PP): Non-absorbable, durable, widely used.
- Polyester (PET): Slightly less durable than PP, used in coated composites.
- Expanded Polytetrafluoroethylene (ePTFE): Soft, microporous, reduced tissue ingrowth.
- Polyvinylidene fluoride (PVDF): Newer material, biocompatible, less prone to shrinkage.
- Absorbable synthetic meshes: Polyglycolic acid (PGA), polyglactin (Vicryl), and polydioxanone (PDO).
Biological Mesh Types
- Autologous grafts: Harvested from the patient’s own tissue, such as fascia lata or dermis.
- Allografts: Human donor-derived acellular dermal matrices (ADMs).
- Xenografts: Derived from porcine or bovine sources, processed to remove cellular components.
Autologous: Refers to grafts made from the patient’s own tissue, such as fascia or skin. These grafts carry a low risk of immune rejection and eliminate the chance of disease transmission, since the material is already part of the patient’s body. However, they typically require longer operative times due to the need to harvest the tissue and may result in complications or discomfort at the donor site.
Heterologous: Refers to biological grafts obtained from a donor that is not the patient, and they can be further categorized as:
Allografts: Grafts sourced from human donors (e.g. cadaveric skin or fascia).
Xenografts: Grafts derived from other species, most commonly porcine (pig) or bovine (cow) tissue. These are more readily available but they may elicit immune responses. These grafts are processed to remove cellular material in a step called decellularisation, which reduces the risk of immune rejection while preserving the structural integrity needed for tissue support. The benefits of heterologous grafts include immediate availability, reduced operative time, and avoidance of donor site morbidity. However, they may elicit immune responses, carry a theoretical risk of disease transmission (although minimal with modern processing techniques), and show variability in long-term integration and resorption rates. Xenografts in particular may be more prone to early degradation or inflammatory responses, depending on the degree of crosslinking and sterilisation method used.
Manufacturing of Mesh Products
Meshes are manufactured via knitting or weaving techniques, where synthetic meshes are typically extruded and knitted into different pore sizes and densities to meet the specific needs of the procedure. These meshes can vary in weight, elasticity, and pore size, each chosen based on the surgical requirement. The pore size is an essential factor in allowing fibroblast migration and tissue integration, with larger pores facilitating better cell infiltration but potentially compromising strength, and smaller pores providing more support but possibly limiting tissue ingrowth. Synthetic materials like polypropylene or ePTFE are used to create meshes with specific properties, such as higher durability or biocompatibility. Biological meshes, on the other hand, undergo a process called decellularisation, which involves removing cellular components to reduce immunogenicity and ensure that the mesh will not provoke an adverse immune response in the recipient. Following decellularisation, biological meshes are sterilized to eliminate pathogens while retaining the essential structural integrity required for optimal tissue integration. This ensures the mesh supports tissue healing while minimising risks such as rejection, infection, or inflammation.
Composite Meshes
Composite meshes combine multiple materials to balance strength and biocompatibility. Typically, a synthetic core is paired with an absorbable or biologically inert coating to minimize adhesion and enhance integration.
Thus, mesh design is a balancing act: optimal designs promote robust tissue repair while minimising complications, such as infection, chronic pain, or erosion into surrounding tissues.
MESH – THE GOOD AND THE BAD
Recent Mesh Controversies
Synthetic vaginal meshes were implicated in chronic pain, erosion, and organ damage. High-profile legal cases and patient advocacy led to increased scrutiny, recalls, and regulatory bans. Countries including the UK, Australia, and New Zealand imposed moratoria on transvaginal mesh used for pelvic organ prolapse due to safety concerns, pending further review and approval processes. The UK first issued a temporary suspension on the use of transvaginal mesh in July 2018, following increasing reports of complications. Australia followed with its own suspension in 2018, calling for a review of the safety and efficacy of mesh implants, particularly for pelvic organ prolapse surgeries. New Zealand’s moratorium, issued in 2018 as well, restricted the use of transvaginal mesh while further investigation was conducted into the risks involved. These measures have led to ongoing regulatory reviews and reconsideration of guidelines for mesh use.
There were many factors that contributed to these problems experienced by some patients (mostly women). Many patients had excellent results from their mesh-based surgery and it is important to remember that the moratorium was put in place because there were too many patients – though still a small minority – who had an awful outcome. Contributory factors included the inadvertent contamination of the sterile mesh when it was placed internally, poor training of some surgeons permitted to use mesh, a lack of governance processes to identify and sanction surgeons who had poor results and a lack of transparency by the profession.
Consequently, the more widespread use of meshes by other specialties was put in the spotlight. Whilst no specialty has a zero rate of mesh complications, the perception amongst surgeons in these specialties was that mesh was not a problem. Nonetheless, an industry-wide process of audit and careful scrutiny by regulatory authorities, scientific committees, Colleges of Surgeons (registration bodies), patient advocacy groups and other stakeholders was undertaken. Set out below are the summary findings from many of these groups.
Guidelines and Recommendations on mesh use from around the world:
– Gynaecological Surgery
- USA: ACOG and AUGS recommend restricted mesh use in prolapse; stress incontinence mesh may be used in selected cases.
- UK: NICE recommends non-mesh treatments as first line for prolapse; mesh slings for incontinence may be used with informed consent.
- Australia: TGA suspended transvaginal meshes; RANZCOG supports non-mesh options.
- New Zealand: Medsafe follows TGA; mesh registry and surgeon accreditation recommended.
– Colorectal Surgery
- USA: The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recommends the use of synthetic mesh in clean, non-contaminated surgical fields. They endorse the use of permanent synthetic meshes, such as polypropylene, as the standard of care for hernia repairs in these conditions. SAGES emphasises that when there is no risk of infection or contamination, synthetic mesh offers the best outcomes in terms of strength, durability, and reducing the recurrence of hernias. They also recommend that the surgeon’s technique, patient selection, and proper post-operative care are critical factors in achieving the best results. However, SAGES, like other organisations, stresses the importance of caution in contaminated fields, where synthetic mesh may be avoided, and biologic or absorbable meshes may be considered. The American Society of Colon and Rectal Surgeons (ASCRS) advises caution in the use of mesh in contaminated or potentially contaminated fields. Their Clinical Practice Guidelines (2021) state: “In the presence of contamination or infection, the use of permanent synthetic mesh should generally be avoided due to a higher risk of mesh infection and complications. Biologic or absorbable mesh may be considered in these settings.” The ASCRS emphasizes patient selection and surgical technique as critical factors for minimising complications when mesh is deemed necessary in such environments.
- UK: The Association of Coloproctology of Great Britain and Ireland (ACPGBI) recommends the use of biologic mesh in infected or potentially contaminated surgical fields. In its position statement on rectal prolapse and pelvic floor disorders, the ACPGBI states: For clean, non-contaminated surgical fields, the ACPGBI and other UK professional organizations support the use of synthetic mesh, such as polypropylene, for hernia repairs and pelvic surgeries, where its durability and reduced recurrence rates offer substantial benefits. “In the presence of infection or significant contamination, biologic mesh should be preferred over synthetic materials due to a lower risk of infection and erosion.” The organisation supports individualised patient care with careful consideration of the risks and benefits, emphasising that “a biologic mesh may provide adequate reinforcement without the long-term complications associated with synthetic mesh in septic environments.”
- Australia/New Zealand: The Royal Australasian College of Surgeons (RACS) and the Colorectal Surgical Society of Australia and New Zealand (CSSANZ) jointly recommend biologic mesh in contaminated settings, such as rectal prolapse with infection. For clean, non-contaminated surgical fields, the Royal College of Surgeons of England (RCS) and other Australian/New Zealand professional organizations recommend synthetic mesh, particularly polypropylene, as the preferred choice for hernia repairs and abdominal wall reinforcement due to its strength and durability. Their guidelines state: “In the presence of infection or significant contamination, synthetic mesh should be avoided due to increased risks of erosion and infection. Biologic meshes provide a safer alternative with acceptable reinforcement properties in such scenarios.” These recommendations are consistent with broader safety reviews by the Therapeutic Goods Administration (TGA), which has called for cautious mesh use and enhanced patient surveillance.
– Urology Surgery
- USA: AUA supports mid-urethral slings under strict guidelines.
- UK: BAUS and NICE call for limited use and patient registries.
- Australia/New Zealand: Use restricted; robust informed consent mandated.
– Hernia Surgery
- USA: SAGES supports synthetic mesh in most hernia repairs.
- UK: Royal College of Surgeons of England endorses synthetic mesh with surveillance.
- Australia/New Zealand: Synthetic use encouraged under HerniaSurge guidelines.
To expand a little more on the subject of abdominal hernia repair with mesh:
Synthetic meshes are endorsed for abdominal wall hernia repairs due to reduced recurrence rates and improved long-term outcomes. This endorsement is substantiated by leading surgical authorities:
- SAGES (USA): The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) states that permanent synthetic mesh materials, especially polypropylene, remain the standard of care in most abdominal wall hernia repairs. Their guidelines highlight reduced recurrence and durability as major benefits of synthetic mesh use.
- HerniaSurge Group (Global): The HerniaSurge international consensus guidelines emphasise that synthetic mesh repair significantly reduces recurrence compared to non-mesh repairs. They advocate for synthetic mesh as the primary option in most hernia repairs, barring contaminated fields.
- Royal College of Surgeons and British Hernia Society (UK): In a joint statement, the RCS and BHS reaffirm that mesh-based inguinal hernia repair remains a safe and effective option, with low recurrence rates and acceptable risk profiles. They also support enhanced patient consent and long-term outcome surveillance through hernia registries.
“What about mesh used in rectal prolapse surgery, especially in women? Is this not the same as gynaecology or urology?” you might well ask. The answer is “No, they are not the same situation”. Let’s look at some scientific scrutiny of the principal operation for rectal prolapse that requires mesh, called Ventral Mesh Rectopexy (VMR):
Outcomes for rectal prolapse surgery by keyhole surgery or robotic VMR using mesh are as follows:
- Length of Stay: Generally 2–4 days
- Complications: Mesh erosion (rare, <1% incidence based on D’Hoore et al., 2004 and follow-up studies), infection (<2% as reported in multiple cohort analyses), and constipation resolution (~40–60%). These statistics are drawn from several high-quality studies and systematic reviews. For example, Smart et al. (2020) conducted a systematic review of ventral mesh rectopexy and found low complication rates, with mesh-related erosion occurring in less than 1% of cases and infection rates under 2%. Constipation resolution rates ranged from 40% to 60%, depending on the study population and follow-up duration. These findings are corroborated by long-term observational data and are consistent across laparoscopic and robotic-assisted cohorts.
- Functional Outcomes: Improved continence and evacuation are consistently reported following laparoscopic and robotic ventral mesh rectopexy. Large cohort studies and meta-analyses show significant functional benefits: for example, continence improved in 60–80% of patients, with fecal incontinence scores such as the Wexner score decreasing by an average of 3–5 points postoperatively. Evacuation difficulties improved in 50–75% of patients, with many reporting relief from obstructed defecation syndrome (ODS). One systematic review (Smart et al., 2020) indicated that up to 70% of patients experienced resolution or significant improvement in ODS symptoms within 6 months. These improvements are supported by both objective scoring systems and subjective patient-reported outcomes.
- Quality of Life (QoL) Assessments: SF-36 (Short Form-36) and GIQLI (Gastrointestinal Quality of Life Index) scores significantly improved. The SF-36 assesses eight health domains, including physical functioning, bodily pain, general health perceptions, vitality, social functioning, role limitations due to physical and emotional problems, and mental health. The GIQLI is a disease-specific instrument evaluating symptoms, emotional and physical function, social integration, and treatment response in patients with gastrointestinal disorders. Improvements in these scores indicate better overall quality of life and functional outcomes following ventral mesh rectopexy.
- Patient-Reported Outcomes Measures (PROMs): High satisfaction rates have been reported consistently in large cohort studies, with over 80% of patients expressing improvement in symptoms, reduced recurrence, and willingness to recommend the procedure. Patient-reported outcome measures (PROMs) capture subjective assessments of bowel function, continence, pain levels, and daily quality of life following ventral mesh rectopexy. Tools such as visual analogue scales (VAS) and condition-specific questionnaires often complement the SF-36 and GIQLI, offering multidimensional insights into the patient’s postoperative experience and satisfaction.
IN SUMMARY
Modern surgical mesh has simply revolutionised the repair of many weakened structures in the human body and it remains a key asset to the surgeon in restoring your anatomy, your function and your health. A good grasp of what mesh actually is, how it works and how carefully it has been independently regulated will build your confidence when proceeding with surgery. All mesh operations that I undertake in my speciality of Colorectal and General Surgery are strongly endorsed by our industry regulators and professional bodies from around the world, thanks to the intense scrutiny of their safety and efficacy. You will always have the option to discuss mesh issues prior to your surgery with me, or to opt out of a mesh-based operation – and without prejudice.
Bibliography
- American College of Obstetricians and Gynecologists (ACOG) – https://www.acog.org/
- American Urogynecologic Society (AUGS) – https://www.augs.org/
- National Institute for Health and Care Excellence (NICE) – https://www.nice.org.uk
- Therapeutic Goods Administration (TGA) – https://www.tga.gov.au/
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) – https://ranzcog.edu.au
- Medsafe New Zealand – https://www.medsafe.govt.nz
- American Society of Colon and Rectal Surgeons (ASCRS) – https://fascrs.org
- Association of Coloproctology of Great Britain and Ireland (ACPGBI) – https://www.acpgbi.org.uk
- American Urological Association (AUA) – https://www.auanet.org
- British Association of Urological Surgeons (BAUS) – https://www.baus.org.uk
- Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) – https://www.sages.org
- Royal College of Surgeons of England – https://www.rcseng.ac.uk
- HerniaSurge Group Guidelines – https://www.herniasurge.com
- D’Hoore A, Penninckx F. Laparoscopic ventral rectopexy. Surg Endosc. 2004.
- Smart NJ et al. Ventral mesh rectopexy: a review. Colorectal Dis. 2020.
- van Iersel JJ et al. Robotic ventral mesh rectopexy: meta-analysis. Int J Colorectal Dis. 2021.